This post has already been read 15860 times!
There is growing recognition within pharma that inventory management should be a top priority.
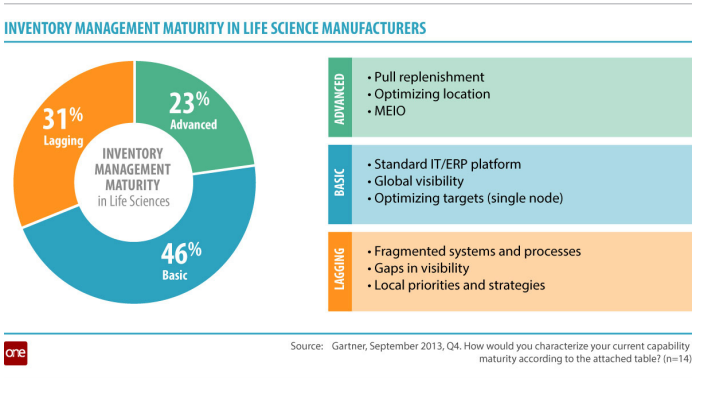
Industry insiders are beginning to put increasing focus on improving their inventory management practices, which lag far behind those of other industries. A recent Gartner survey found that 77% of life sciences manufacturers have reached an inventory management maturity that is only basic or lagging.
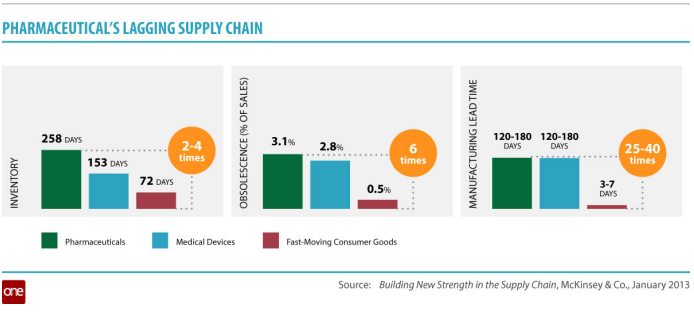
Unlike the consumer goods sector, the stock levels in the pharmaceutical supply chain (“pipeline stocks”) typically amount to 30–90% of annual demand in quantity. There are usually 4–24 weeks of finished good stocks. Stock inventory typically ranges between 1 and 8 turns. And supply chain cycle times (defined as elapsed time between material entering as raw material and leaving as product) are often between 1 and 11 months. Expired product is certainly very costly and the returns and destruction process difficult to manage from a compliance perspective. This represents a significant opportunity from an inventory perspective as compared to other industries per the chart below.
The requirement for increased supply-chain visibility will be highest in the generics and OTC segments, where cost pressures are especially strong, as well as in the specialty drugs segment, where inventories tie up large amounts of capital due to the high product values involved. For specialties, a direct-distribution model with a single regional or global distribution center generating visibility to multiple points of sale could be an early win.
The optimization of supply chains could lead to an increased risk of stock-out situations, ranging from local, short-term shortages to regional drug shortages, due to manufacturing issues that require dedicated shortage-management efforts. Increased visibility of inventories will be both essential and very useful. Potential excess supplies of product in one place can be moved in time to prevent supply shortages in other places. The same would be true in a local epidemic or even pandemic scenario, when demand for a specific product suddenly increases.
Moving from a traditional supply chain design to a cloud-based planning and execution platform can provide the framework and architecture to optimize supply chain performance while minimizing any associated risks.
If you like this topic, I suggest you read, Intelligent Control Towers for Pharmaceutical and Medical Device Supply Chains, which explains the basics in more detail.
- Map of U.S. Trucking Spills in 2016 - January 13, 2017
- What is aPaaS? A Way to Supercharge Your App Development - December 12, 2016
- Future of Transportation: Goodyear’s Radical Smart Tire Concept - November 3, 2016